TrialLytics
Triallytics: Statistical Platform for Clinical Trials
Project Overview
Triallytics is a powerful and user-friendly statistical platform designed to streamline the analysis of clinical trial data. Built with a focus on flexibility and scientific rigor, Triallytics offers tools to perform advanced statistical analyses such as mixed models, Cox regression, and more. It enables clinical researchers and statisticians to extract meaningful insights from their data, ensuring the accurate and reliable evaluation of treatments and interventions.
The platform supports a variety of clinical trial designs and offers customizable workflows that can be adapted to specific trial requirements. Whether you're working on survival analysis, longitudinal studies, or multi-group comparisons, Triallytics provides an intuitive interface to make complex analyses straightforward.
Key Features
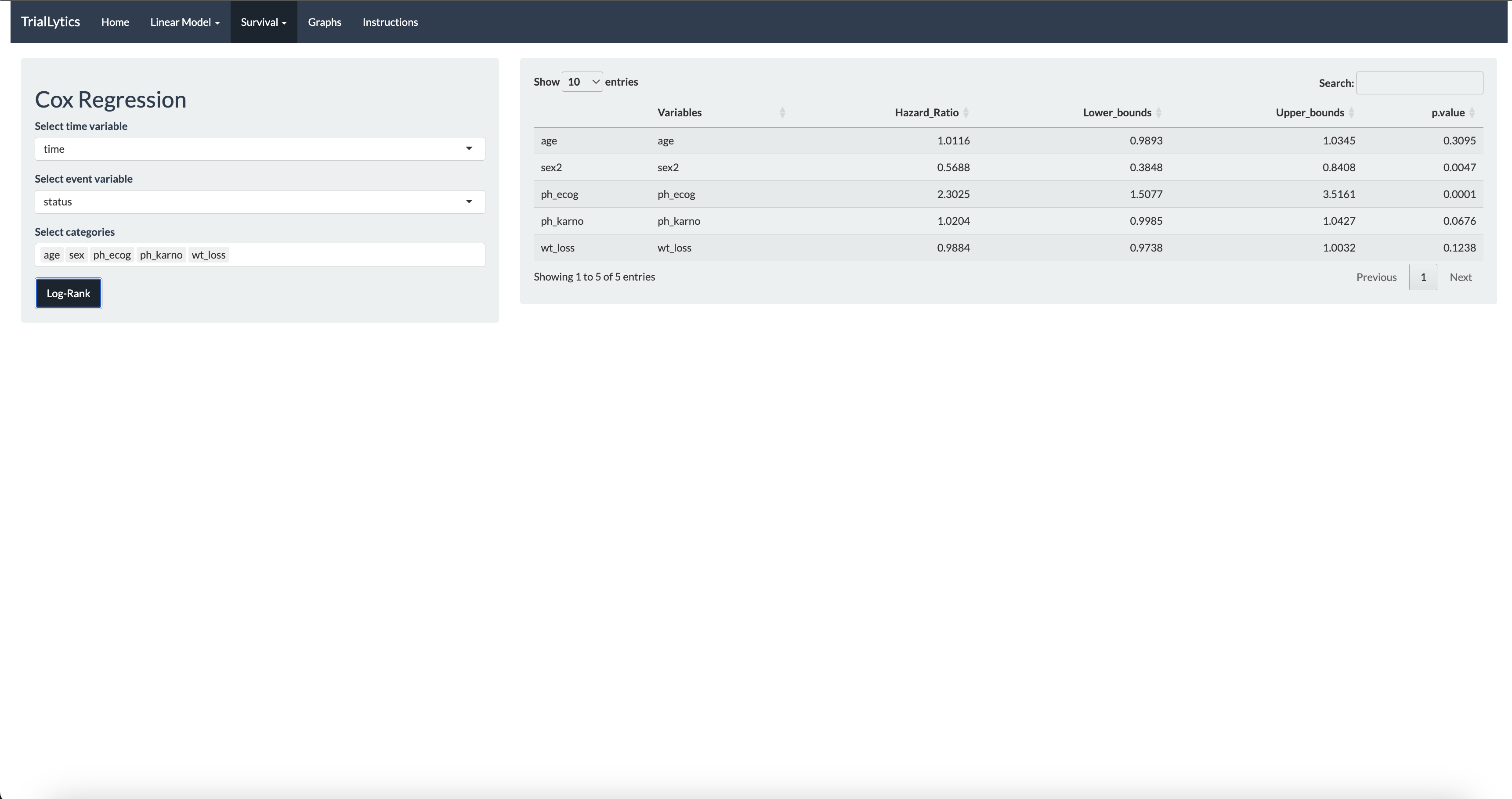
- Comprehensive Statistical Tools: Supports key statistical methods used in clinical trials, such as mixed models, ANOVA, Cox regression, and linear regression.
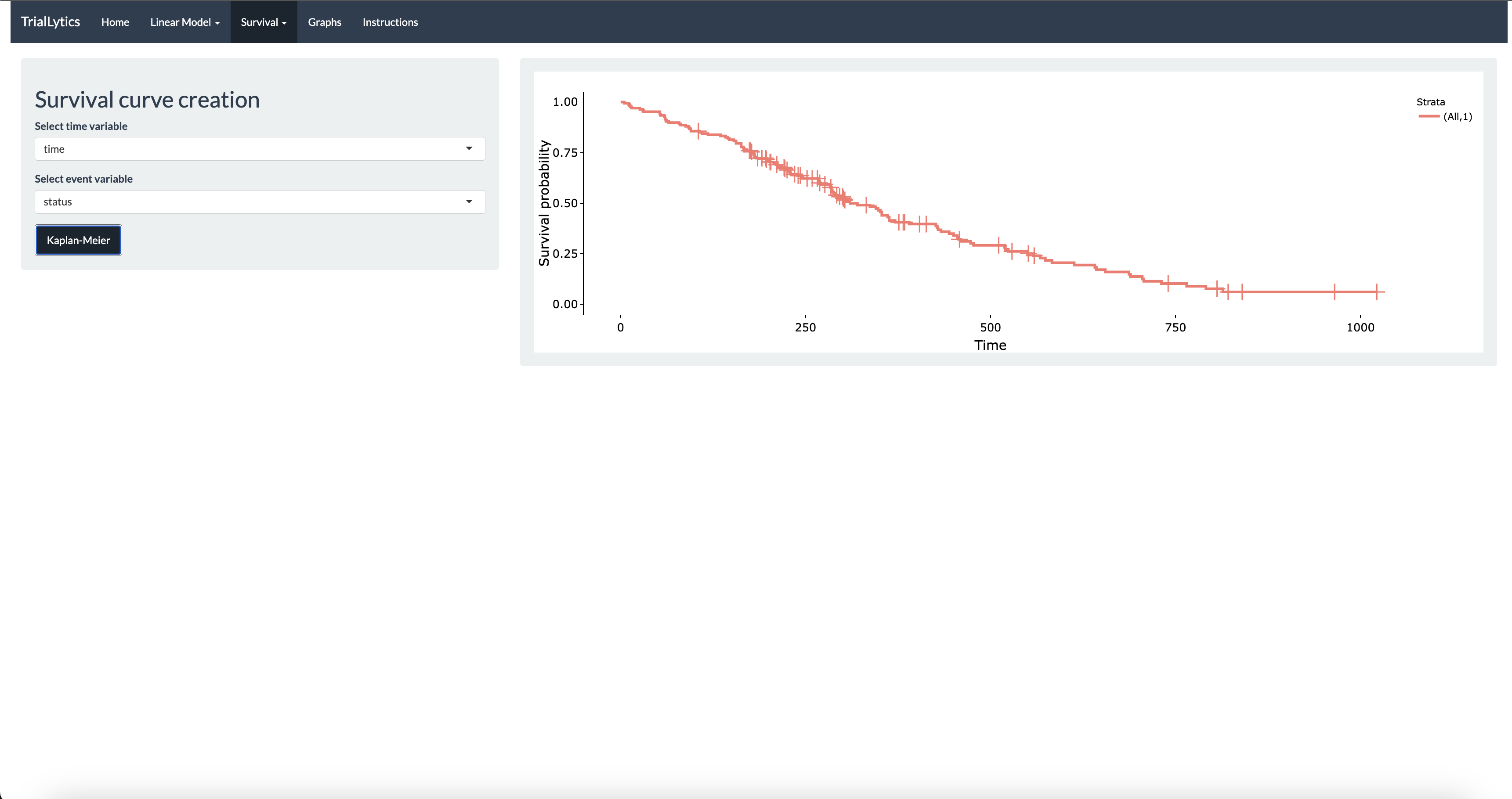
- Hazard Ratio Interpretation: Provides hazard ratio outputs for Cox models, offering an easy way to interpret the impact of treatment variables on time-to-event outcomes.
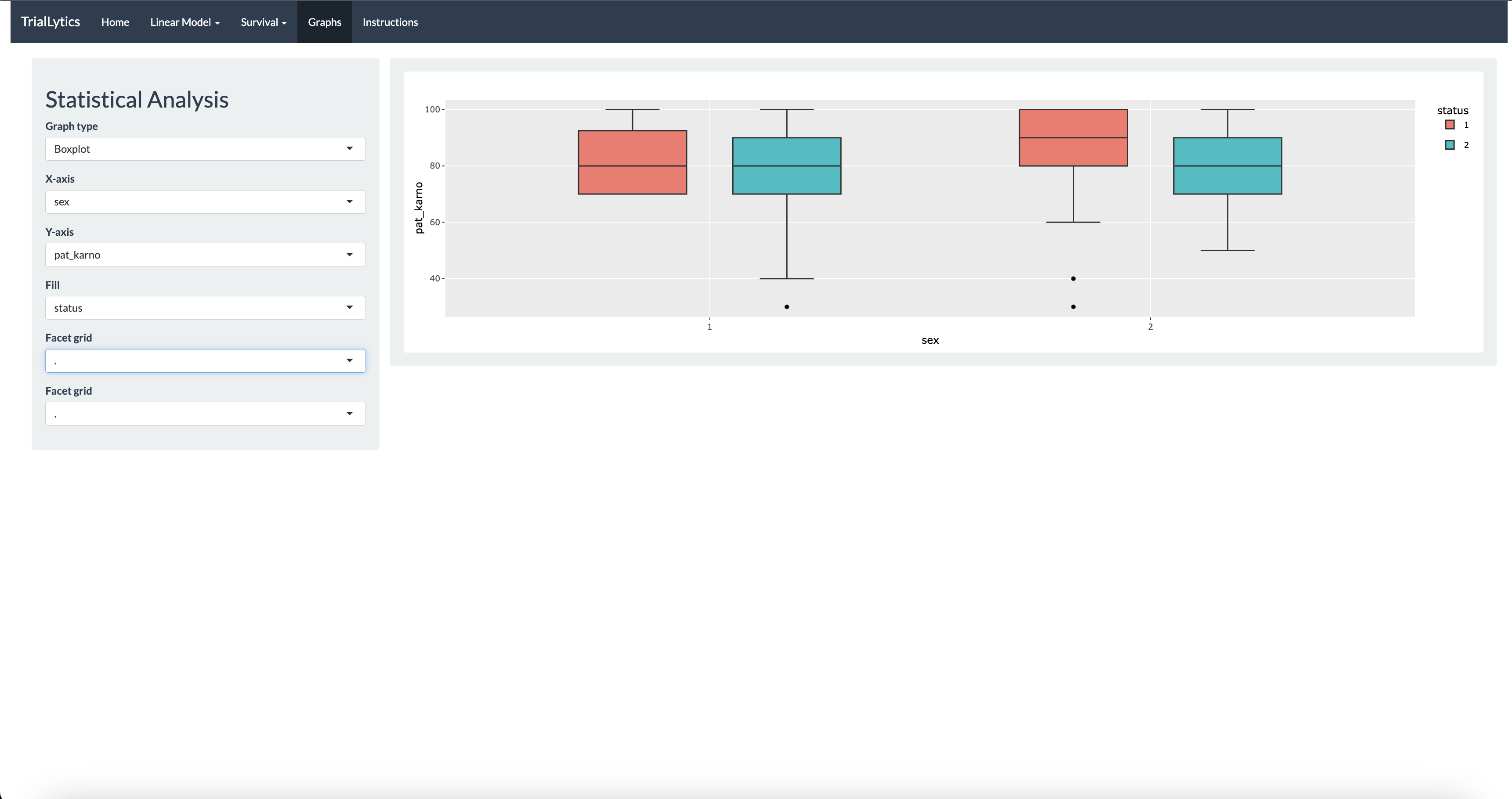
- Interactive Data Visualizations: Powered by Plotly, the platform provides dynamic and customizable charts to visualize results in real-time.
- RShiny Integration: Built using RShiny, the platform allows for the development of web-based applications for easy interaction with statistical models and results.
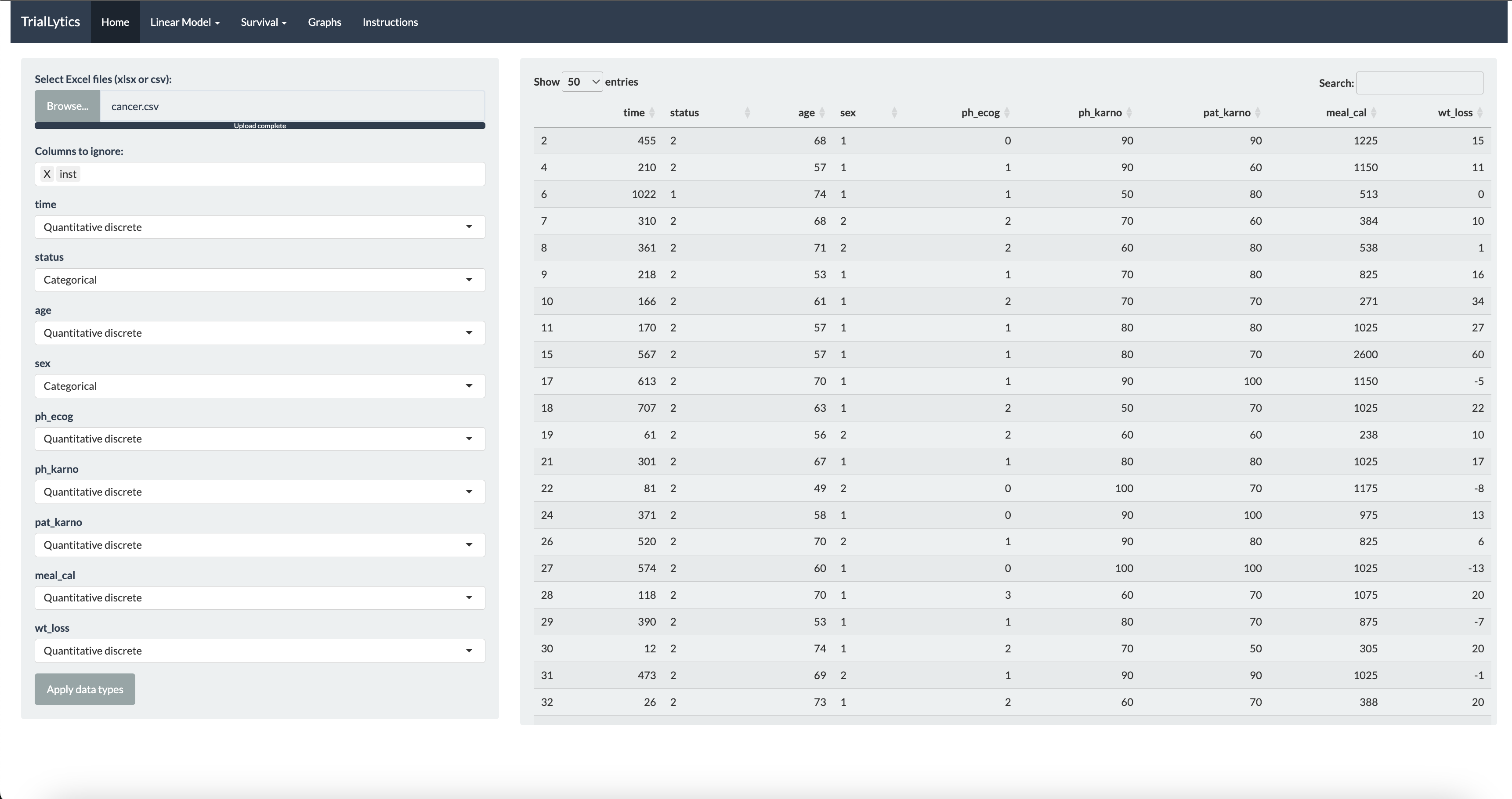
- Advanced Imputation Strategies: Handles missing data with sophisticated imputation methods to ensure robustness in results.
Technologies Used
- R: Core language for the platform's statistical computations and model building.
- RShiny: Provides the interactive web application interface for users to run analyses and visualize results.
- Plotly: Enables interactive, customizable visualizations of the statistical results.
- Docker: Ensures portability and scalability by containerizing the platform for seamless deployment across various environments.
- Emmeans, Lme4, Survival Packages: Used for mixed models, survival analysis, and other statistical methods relevant to clinical trials.
Scientific and Technical Details
Triallytics implements a variety of advanced statistical methods tailored for clinical trials. These include:
- Mixed Models: Useful for analyzing repeated measures or clustered data, where responses may not be independent.
- Cox Regression: Provides time-to-event analysis and calculates hazard ratios to assess the impact of covariates on survival time.
- ANOVA and Linear Models: For comparing means across multiple groups and assessing the impact of continuous variables on an outcome.
- Imputation: Sophisticated techniques to handle missing data, ensuring that the analysis remains robust and unbiased.
Applications
The Triallytics platform can be applied to:
- Cosmetic Efficacy Testing: Evaluate the efficacy of cosmetic products, such as the long-wear of lipstick or blush, by using mixed models to account for repeated measures and individual variability.
- Clinical Trials: Analyze time-to-event data in clinical trials with Cox regression models, ensuring accurate hazard ratio interpretations to assess treatment effects.
- Pharmaceutical Research: Provide robust statistical methods for analyzing drug trials, ensuring regulatory compliance and rigorous statistical validation of results.
- Data Visualization and Reporting: Create interactive reports for stakeholders, allowing them to explore trial results dynamically with intuitive graphical interfaces.
Conclusion
Triallytics is a versatile and powerful statistical platform that simplifies the analysis of complex clinical trial data. By combining advanced statistical models with interactive data visualization, the platform offers a comprehensive solution for clinical researchers. Docker integration ensures that Triallytics is both portable and scalable, making it an ideal tool for analyzing clinical trials of any size.
Project information
- Category Statistics
- Project date September 2024
- Project URL triallytics.mortreau.net
- Visit Website